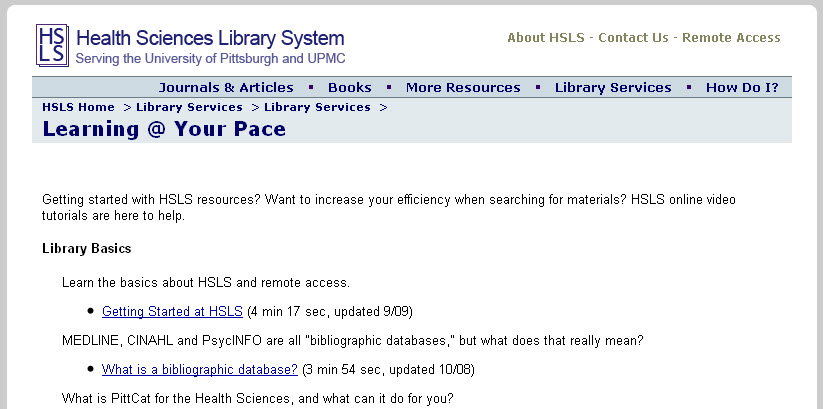
The year 2010 marks the 10th anniversary of the completion of the draft human genome sequence. During the past decade, sequencing technology has vastly improved, thereby significantly decreasing the cost of sequencing an organism’s entire genome. The initial project cost three billion dollars and 13 years of effort by several high-powered international laboratories. Today the cost for sequencing a complete human genome is approximately $40,000. In the not-too-distant future, one lab technician using one machine will be able to sequence a complete human genome in one day for $1,000 or less.
Empowered by these technological advances, scientists now have the ability to rapidly compare the genetic alphabets of groups of people that show a particular trait with those that don’t, and thus to identify genetic variations associated with a disease or condition. This effort has created a list of biomarkers linked to various diseases and conditions, such as smoking addiction or resistance to HIV/AIDS. The availability of a rapidly growing list of genetic biomarkers, coupled with the rapidly decreasing cost of genome sequencing and genotyping, has given birth to two new fields—personal genomics and personalized medicine.
Direct-to-consumer genome retail companies, such as 23andMe, Navigenics, and deCODEME, offer the latest in genotyping technology to the general public for $500 to $2,000. Now anyone can order a kit online from one of these companies, spit into the provided tube, and send it back to the company’s lab for DNA analysis. The results are available online within 6-8 weeks.
Access to your personal genome enables you to identify genetic risk factors or disease markers you may carry, as well as determine whether your children are susceptible to an inherited condition such as cystic fibrosis or sickle cell anemia. Knowing your genetic predisposition for certain diseases or conditions can also help motivate you to take steps towards a healthier lifestyle.
The greatest promise of personal genome information is its potential to offer individualized drug treatment. People metabolize drugs at different rates; however, physicians often prescribe drugs and select dosages based on a “one-size fits all” paradigm. Patients who metabolize a particular drug more slowly than others might need a lower dose and thus could experience adverse side effects if prescribed the standard dosage. Knowledge of your own genetic make-up will help you and your doctor choose the best medications at the dosage level that is the safest and most effective for you.
Despite the promise of personal genomics and personalized medicine for improving treatment practices and overall health, there is active debate within the medical profession and the public about the appropriateness of their use. Modern science indicates that three components comprise our health conditions: our own genetic make-up, the genetic make-up of all the microbial organisms that live in our bodies, and interactions with our environment. Knowledge of one’s personal genome casts light onto only one-third of this equation, and therefore does not provide a complete picture of genetic risks.
Use of the personal genome also raises ethical concerns. If someone has access to an individual’s genetic profile, it could affect decisions made regarding that person, such as denial of employment, disability insurance, or life insurance. The Genetic Information Non-discrimination Act of 2008 protects against discrimination by health insurers and employers on the basis of DNA information.
Considering both the benefits and risks of personal genomics and personalized medicine, it is important for everyone to educate themselves on these topics. Two authoritative resources include the Personal Genome Project and the Personalized Medicine Coalition.
~ Ansuman Chattopadhyay and Carrie Iwema
 Please plan to attend the upcoming HSLS-sponsored Mobile Computing Workshop on Friday, October 29, 2010. Featured speakers include Dr. Roman M. Cibirka, vice president for Instruction and Enrollment Management and associate provost at the Medical College of Georgia. Dr. Cibirka has been internationally recognized for his achievements in mobile technologies, virtual simulation and game-based educational innovations. Other speakers are Iltifat Husain, founder and editor-in-chief of iMedicalApps.com and UPMC’s Dr. Rasu B. Shrestha, medical director, Interoperability and Imaging Informatics. Afternoon break-out sessions are geared for both novice and expert users, with topics such as using HSLS’s mobile resources, mobile technology for dummies, and mobile applications that pertain to the field of molecular biology.
Please plan to attend the upcoming HSLS-sponsored Mobile Computing Workshop on Friday, October 29, 2010. Featured speakers include Dr. Roman M. Cibirka, vice president for Instruction and Enrollment Management and associate provost at the Medical College of Georgia. Dr. Cibirka has been internationally recognized for his achievements in mobile technologies, virtual simulation and game-based educational innovations. Other speakers are Iltifat Husain, founder and editor-in-chief of iMedicalApps.com and UPMC’s Dr. Rasu B. Shrestha, medical director, Interoperability and Imaging Informatics. Afternoon break-out sessions are geared for both novice and expert users, with topics such as using HSLS’s mobile resources, mobile technology for dummies, and mobile applications that pertain to the field of molecular biology.

 Libraries, by definition, are organized operations. We try to keep track of everything we do. We count our virtual and in-person visits, we count our loans, we count the number of reference questions received, and we count the number of educational sessions offered. We analyze whether these numbers are going up or down, and we scratch our heads to figure out what it all means.
Libraries, by definition, are organized operations. We try to keep track of everything we do. We count our virtual and in-person visits, we count our loans, we count the number of reference questions received, and we count the number of educational sessions offered. We analyze whether these numbers are going up or down, and we scratch our heads to figure out what it all means.
 Robin Sencenbach earned her BA in English, with a minor in history, at Allegheny College. While at Allegheny College, she interned at the library where she helped move and organize special collections materials during the library’s renovation. Sencenbach also volunteered at the Heinz History Center as a tour guide and intern to the curators. She is interning in the Technical Services Department at Falk Library.
Robin Sencenbach earned her BA in English, with a minor in history, at Allegheny College. While at Allegheny College, she interned at the library where she helped move and organize special collections materials during the library’s renovation. Sencenbach also volunteered at the Heinz History Center as a tour guide and intern to the curators. She is interning in the Technical Services Department at Falk Library. Priya Shenoy received her BS in nursing from the University of Pennsylvania. Shenoy worked as a staff nurse on a cardiac floor at Georgetown University Hospital and as a public health nurse. As an undergraduate, she worked in the circulation department at the University of Pennsylvania’s Charles Patterson Van Pelt Library. Before deciding to pursue a career in library science, Shenoy shadowed librarians at the National Library of Medicine, George Washington University’s Himmelfarb Health Sciences Library, and a public library. This past summer, she interned at Georgetown University’s Dahlgren Memorial Library, where she spent time in every department. Shenoy is interning in the Reference Department at Falk Library.
Priya Shenoy received her BS in nursing from the University of Pennsylvania. Shenoy worked as a staff nurse on a cardiac floor at Georgetown University Hospital and as a public health nurse. As an undergraduate, she worked in the circulation department at the University of Pennsylvania’s Charles Patterson Van Pelt Library. Before deciding to pursue a career in library science, Shenoy shadowed librarians at the National Library of Medicine, George Washington University’s Himmelfarb Health Sciences Library, and a public library. This past summer, she interned at Georgetown University’s Dahlgren Memorial Library, where she spent time in every department. Shenoy is interning in the Reference Department at Falk Library. Robyn Reed, the 2010-2011 Trainee, earned a BS in medical technology and chemistry from SUNY Fredonia, an MS in pharmacology from SUNY Buffalo, and an MLIS from the University of Pittsburgh. Reed decided to pursue a career in bioinformatics because she’s interested in how computer applications can be utilized to disseminate pertinent information to specific populations. Last summer, Reed completed a consumer health field placement at UPMC Shadyside’s Hopwood Library. She is currently working with Dr. Titus Schleyer in the School of Dental Medicine on a dental informatics project. Reed will spend time in both the Falk Library Reference Department and the Molecular Biology Information Service.
Robyn Reed, the 2010-2011 Trainee, earned a BS in medical technology and chemistry from SUNY Fredonia, an MS in pharmacology from SUNY Buffalo, and an MLIS from the University of Pittsburgh. Reed decided to pursue a career in bioinformatics because she’s interested in how computer applications can be utilized to disseminate pertinent information to specific populations. Last summer, Reed completed a consumer health field placement at UPMC Shadyside’s Hopwood Library. She is currently working with Dr. Titus Schleyer in the School of Dental Medicine on a dental informatics project. Reed will spend time in both the Falk Library Reference Department and the Molecular Biology Information Service.