HSLS has a new online Drug Information Resource Guide, directing users to appropriate resources for different types of drug information.
Online drug resources provided through HSLS range from database compendia to electronic books and primary literature databases. Available database compendia with general drug information include Micromedex, UpToDate, and AccessMedicine. Examples of electronic books include AHFS Drug Information, Meyler’s Side Effects of Drugs, and the King Guide to Parenteral Admixtures.
Resource Highlights
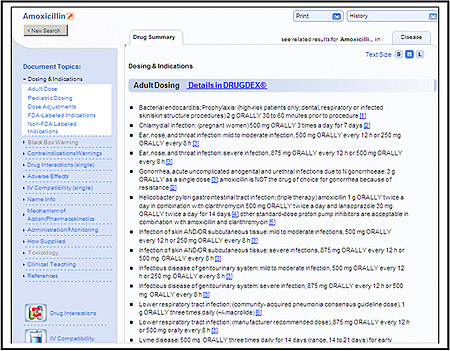
Micromedex Point of Care Interface: to access, click on the link located at the top right of the Micromedex Healthcare Series original homepage.
The simple left menu layout of the interface features the most widely accessed information at the top. This includes dosing, indications, and off label use. Further down, one can easily find information on administration/monitoring which describes how to prepare a drug for administration including stability and dilution, how to administer, and what parameters to monitor. Also on the menu is a “How Supplied” link which provides available formulations of a drug. The Point of Care Interface also has links to detailed drug information from the original DRUGDEX component of Micromedex Healthcare Series. For more information about the Point of Care Interface, see the October 2007 Update article.
FDA Website: a free online resource with drug information for healthcare professionals. Examples of information available through the FDA include the following:
NDC Code Directory: This online directory provides a searchable interface to identify a drug’s National Drug Code (NDC) number. One can also search by NDC to identify a product’s name.
Electronic Orange Book: This online directory provides therapeutic equivalence ratings for generic products.
Drugs@FDA: This database provides searching by drug name, active ingredient, or application number. One can identify all drugs by a specific ingredient or FDA application number, or look up available generics and their therapeutic equivalence. Drugs covered by Drugs@FDA include prescription, over the counter, and therapeutic biologicals.
Drug Alerts: Register for email updates, which provide users with up-to-date information on a variety of topics such as recalls and safety alerts, new drug approvals, and more.
Safety Alerts: Register for email or RSS feed updates for the FDA’s MedWatch Safety alerts
For further assistance on finding drug information please contact Ahlam Saleh, MD, MLS, reference librarian, at saleha@pitt.edu or 412-648-2166.
~ Ahlam Saleh